
Effective as of November 1, 2025! Zinwi Bio Helps You In-depth Interpret South Korea's Tobacco Harm Control Act
On November 1, 2025, South Korea's Tobacco Harm Control Act officially came into force. In fact, this regulation is not an entirely new introduction; its core framework and regulatory direction were initially released several years ago. After multiple rounds of public opinion collection and refinement of detailed rules, it was finally confirmed to be formally implemented this month.

The newly effective regulation sets forth comprehensive and strict regulatory requirements. It not only clearly defines testing standards, submission of compliance documents, and disclosure of product information, but also refines penalties for violations. This establishes a clear compliance threshold for e-cigarette brands entering the South Korean market and imposes higher requirements on the standardized development of the industry.
3.https://www.law.go.kr/LSW/lsSc.do?section=&menuId=1&subMenuId=15&tabMenuId=81&eventGubun=060101&query=%EB%8B%B4%EB%B0%B0%EC%9D%98+%EC%9C%A0%ED%95%B4%EC%84%B1+%EA%B4%80%EB%A6%AC%EC%97%90+%EA%B4%80%ED%95%9C+%EB%B2%95%EB%A5%A0+%EC%8B%9C%ED%96%89%EA%B7%9C%EC%B9%99#undefined
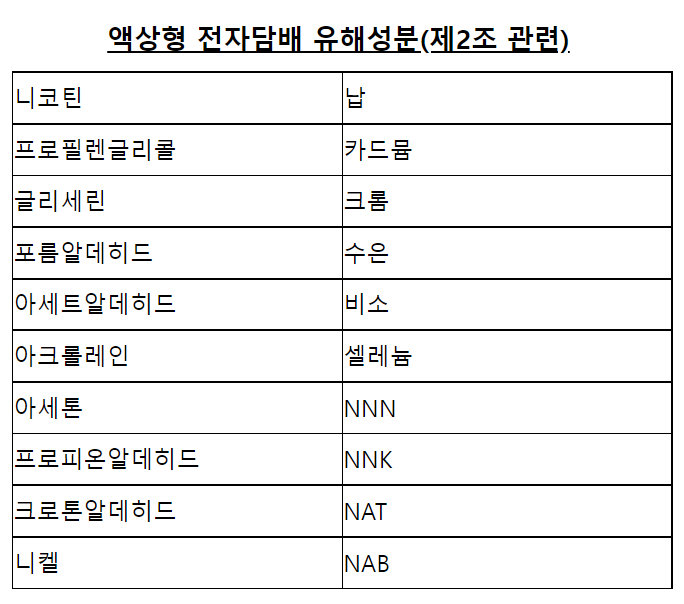
I. E-liquid Ingredients: 20 Ingredients Mandatory for Testing, with Clear Requirements for 3 Main Ingredients

1. Mandatory Testing List and Testing Methods
Testing must be entrusted to institutions accredited by KOLAS (Korea Laboratory Accreditation Scheme). The 20 ingredients are divided into 4 categories, each corresponding to exclusive testing technologies:
https://www.mfds.go.kr/brd/m_209/view.do?seq=44111#none
Zinwi Bio Support: We provide "e-liquid ingredient pre-testing + compliant formula optimization" services. Relying on our CNAS-accredited laboratory (recognized globally), we can conduct screening tests for 20 ingredients on e-liquid samples in advance. For items exceeding standards, we offer formula adjustment solutions (such as replacing flavors with lower risks, optimizing PG/VG ratio) to ensure that the product passes the inspection in one go.
2. Supplementary Explanations on Critical Limits
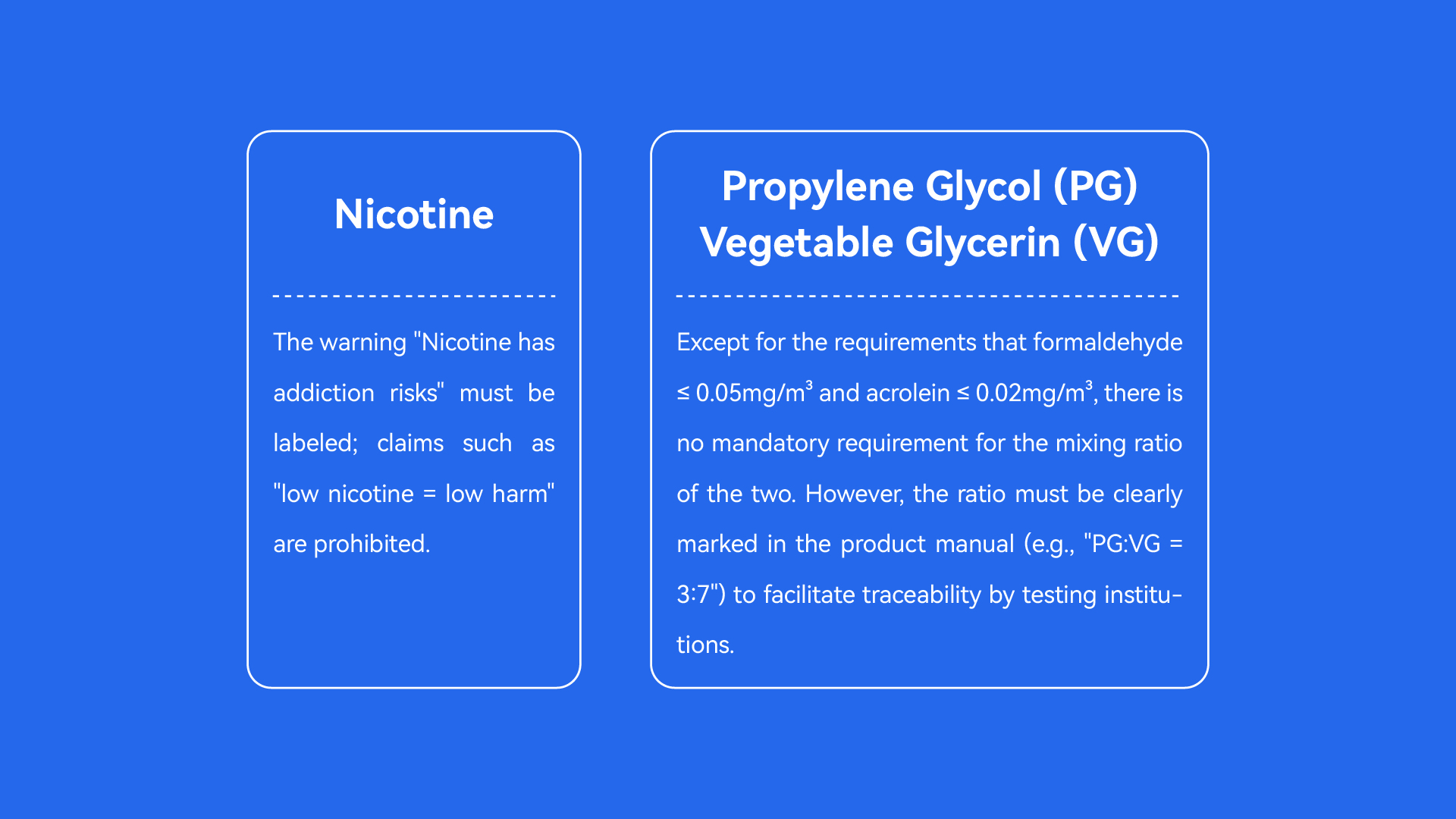
Nicotine: The warning "Nicotine has addiction risks" must be labeled; claims such as "low nicotine = low harm" are prohibited.
Propylene Glycol (PG)/Vegetable Glycerin (VG): Except for the requirements that formaldehyde ≤ 0.05mg/m³ and acrolein ≤ 0.02mg/m³, there is no mandatory requirement for the mixing ratio of the two. However, the ratio must be clearly marked in the product manual (e.g., "PG:VG = 3:7") to facilitate traceability by testing institutions.
Zinwi Bio Support: Both PG and VG used are raw materials that meet U.S. certifications and EU standards. We can provide raw material testing reports from the past 3 months and assist customers in standardizing the labeling of PG/VG ratio in the product manual.
3. Testing Cycle and Report Requirements
Re-testing cycle: A re-test must be conducted every 2 years. The test report must include 4 core pieces of information: "sample number, testing date, qualifications of testers, and KOLAS accreditation mark"—none of which can be missing.
Ingredient change requirements: If there is a change in e-liquid ingredients (such as replacing flavor suppliers or adjusting PG/VG ratio), re-testing must be conducted within 15 working days after the change, and the reasons for the change and impact assessment must be provided.
Zinwi Bio Support: We have established an "e-liquid ingredient change tracking mechanism". If you need to adjust the formula, we can provide a pre-evaluation report for the changed sample within 24 hours, assist in connecting with KOLAS-accredited testing institutions to shorten the re-testing cycle, and draft the "explanation of reasons for ingredient changes" on your behalf to ensure compliance with the requirements of MFDS (Ministry of Food and Drug Safety).
II. Sales and Packaging: Clear Restrictions on Channels and Packaging

1. Detailed Rules for Sales Channels
Offline sales: Only "specialized tobacco stores accredited by KT&G (Korea Tobacco & Ginseng Corporation)" are allowed to sell e-cigarettes. Stores must post the sign "E-cigarettes are only sold to people aged 19 and above" and verify the buyer’s ID (South Korean national ID card or foreigner residence permit).
Online sales: Sales must be conducted through "officially accredited platforms registered with MFDS (Ministry of Food and Drug Safety)". Platforms must set up an "age verification pop-up window" (verification by entering the last 6 digits of the ID card number) and are prohibited from pushing e-cigarette advertisements to minors.
Prohibited activities: "Offline experience and trial" and "purchase-with-gift activities" (e.g., gifting e-cigarette devices when purchasing e-liquid) are prohibited. Only individual products with clearly marked prices can be sold.
Zinwi Bio Support: We can assist in providing "e-liquid product compliance explanation documents" to facilitate the rapid completion of product market access review.
2. Supplementary Requirements for Packaging
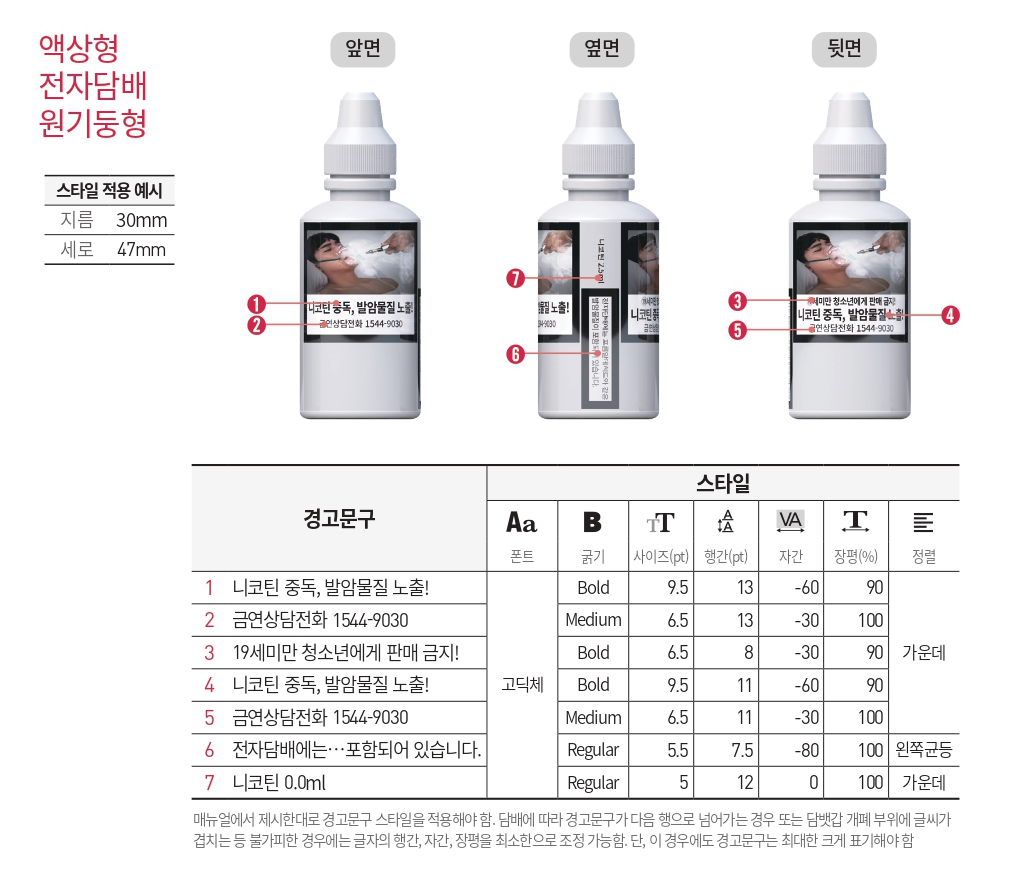
Standardized e-cigarette packaging: Must use "white background + black font". No less than 30% of the package surface area must be used for printing warning messages (e.g., "Long-term use may cause cardiovascular diseases"), and the font height of the warning messages must be ≥ 5mm.
Mandatory labels on packages: Must include "net content of e-liquid, production date, shelf life (≤ 24 months from the production date), and MFDS registration number".
Prohibited promotional terms: Words such as "healthy", "harm-reducing", and "cigarette substitute" are prohibited. Even for nicotine-free e-liquid, only "nicotine-free" can be labeled, and no additional efficacy descriptions are allowed.
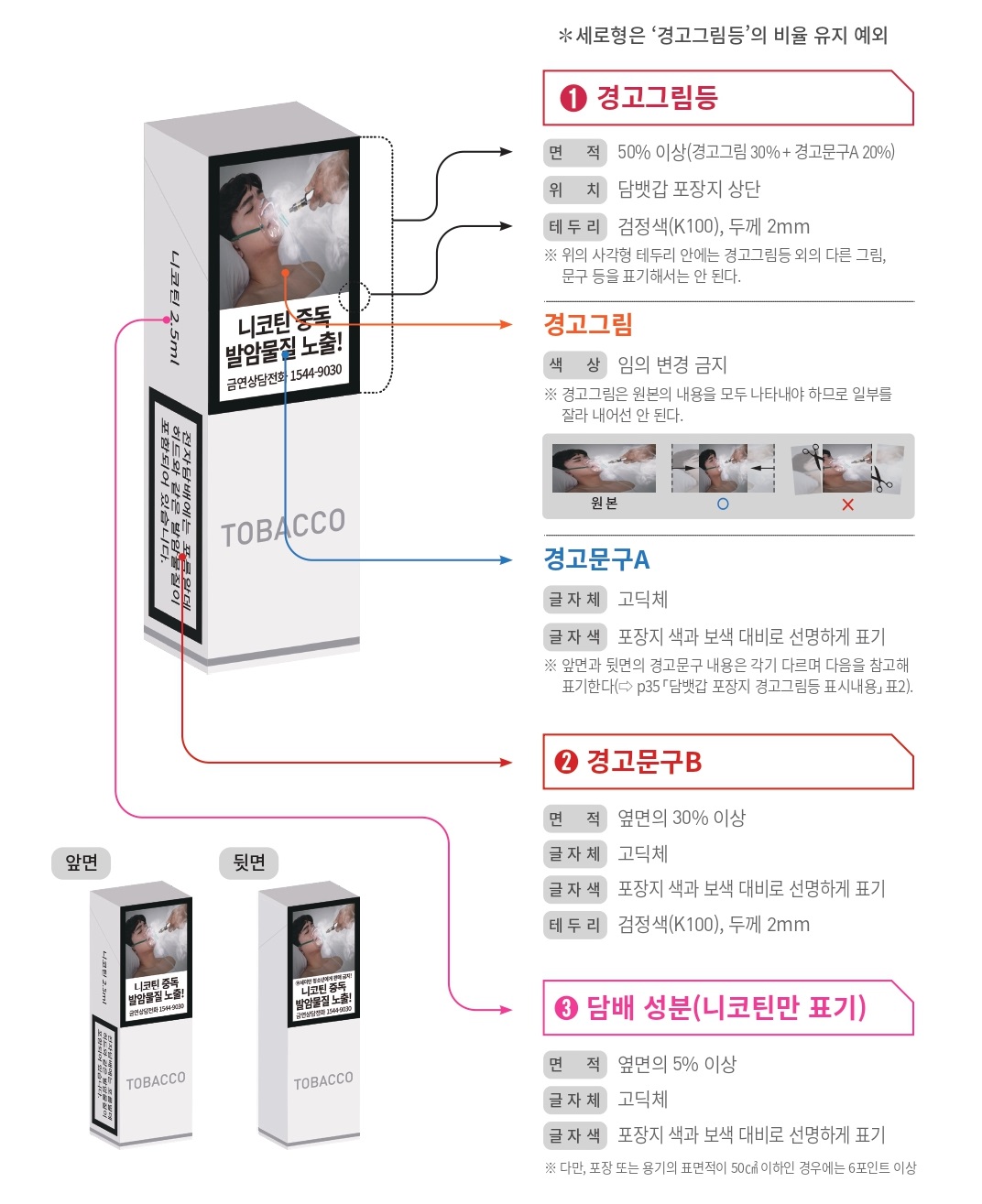

Zinwi Bio Support: We provide a preset "compliant packaging design reference template", and the labeling positions of net content, production date, etc., can be adjusted according to customer needs. Meanwhile, we assist customers in applying for the MFDS registration number and filling in the registration information on their behalf to ensure the standardized format of the registration number.
III. Key Compliance Reminders

1. Details on Raw Materials and Production
E-liquid raw materials: PG/VG used must be "food-grade certified", and suppliers must provide raw material testing reports from the past 6 months.
Nicotine: "Nicotine source certificates" must be retained (e.g., qualification documents of legal tobacco extraction enterprises or synthetic nicotine production enterprises).
Production process: Records of "raw material feeding quantity, mixing temperature, and filling speed" must be kept, with a retention period of ≥ 3 years, for random inspection by MFDS.
Zinwi Bio Support: On the e-liquid raw material side, we provide "full-chain traceability services", enabling traceability of the qualifications of PG/VG and nicotine suppliers as well as their testing reports. On the production side, we use "constant temperature and pressure mixing equipment" to accurately control the mixing temperature. We also provide production records (including feeding quantity and filling speed) that meet the 3-year retention requirement.

2. Specifications for Submission of Compliance Documents
KOLAS test report: Must be submitted to MFDS 30 days before the product is launched on the market. The submission method is "online upload via the MFDS official website + mailing of a paper version (with the company’s official seal affixed)".
Raw material traceability certificate: Must include "supplier name, raw material batch number, and qualification certificate for inspection". If the raw materials are imported, an "customs import declaration form" must be attached additionally.
Production process flow chart: Must mark "critical control points" (e.g., raw material screening, mixing and stirring, aseptic filling) and be signed and confirmed by the company’s technical director.
Summary of required submission materials:

https://www.law.go.kr/LSW/lsSc.do?section=&menuId=1&subMenuId=15&tabMenuId=81&eventGubun=060101&query=%EB%8B%B4%EB%B0%B0%EC%9D%98+%EC%9C%A0%ED%95%B4%EC%84%B1+%EA%B4%80%EB%A6%AC%EC%97%90+%EA%B4%80%ED%95%9C+%EB%B2%95%EB%A5%A0+%EC%8B%9C%ED%96%89%EA%B7%9C%EC%B9%99#AJAX
Zinwi Bio Support: We provide "compliance document packaging services" for e-liquids—assisting customers in organizing KOLAS test reports, raw material traceability certificates (including customs declaration forms for imported raw materials), and production process flow charts (with critical control points marked and signed by our technical director), and helping with online uploads via the MFDS official website, among other services.
3. Detailed Consequences of Non-Compliance
First-time unqualified test result: Rectification and re-testing must be completed within 30 days, and product sales must be suspended during this period.
Second-time unqualified test result: The product must be removed from shelves and recalled, and no new registration applications are allowed within 6 months.
Serious violations (e.g., forging test reports, selling to minors): The enterprise will be included in the "South Korean E-cigarette Industry Blacklist", permanently restricted from market access, and must bear corresponding legal liabilities (with a maximum fine of 100 million won).
Zinwi Bio Support: We have established a "compliance risk early warning mechanism" to track updates to MFDS regulations regularly and remind customers of the re-testing time in advance. If there are issues related to testing, we can provide technical support within 48 hours to assist with re-testing and minimize the impact on product launch.
Zinwi Bio: Win-Win with Professional Compliance Support
South Korea’s Tobacco Harm Control Act ushers in full-cycle compliance supervision, requiring enterprises to meet regulatory standards across raw materials, testing, packaging, and registration. Zinwi Bio offers end-to-end services—including e-liquid pre-testing, formula optimization, raw material traceability, and compliance document packaging—to reduce risks like repeated testing and delayed registration, helping products quickly access the South Korean market. As compliance becomes core competitiveness, we will continue empowering partners with professional capabilities for steady local development.

We will contact you as soon as possible









