
Zinwi Bio's TPD registration service upgraded to help partners travelling in the European market
As the global tobacco industry continues to develop and European market regulations strengthen, registration under the Tobacco Products Directive (TPD) of the European Union has become a necessary step for entering the European market. Zinwi Bio, as a company dedicated to promoting industry compliance, has been assisting clients in completing EU TPD registrations and possesses extensive professional experience in the compliance field.

In recent years, Zinwi Bio's professional compliance team has successfully assisted clients in submitting multiple batches of products for EU TPD registration, helping clients actively address the complex and ever-changing regulatory challenges across multiple European countries, thus reducing risks.
This year, our service has been comprehensively upgraded:
Providing more personalized services: Tailored submission services based on the preferences and needs of our partners.
Improving service quality: Strengthening training for the entire service team to ensure an enhanced quality of service.
Shortening service response time: Optimizing service processes to improve efficiency and reduce waiting times for clients.
Introducing new instruments: Utilizing advanced precision instruments to provide clients with a more intelligent and convenient service experience.
Enhancing after-sales service: Timely resolution of issues during registration to increase customer satisfaction.
We are not only committed to ensuring product compliance but also focus on helping clients understand and comply with complex regulatory requirements. We provide comprehensive compliance support and tailored one-stop solutions to enable partners to confidently explore markets and provide reliable guarantees for the success of their products in the market.
Introduction to Article 20 of the TPD
The European Union's Tobacco Products Directive (TPD) upholds stringent standards for tobacco products and related articles in the market, ensuring quality, safety, and health compliance. Compliance with TPD is mandatory for e-cigarette products intending to enter the EU market for sale, requiring registration six months in advance.
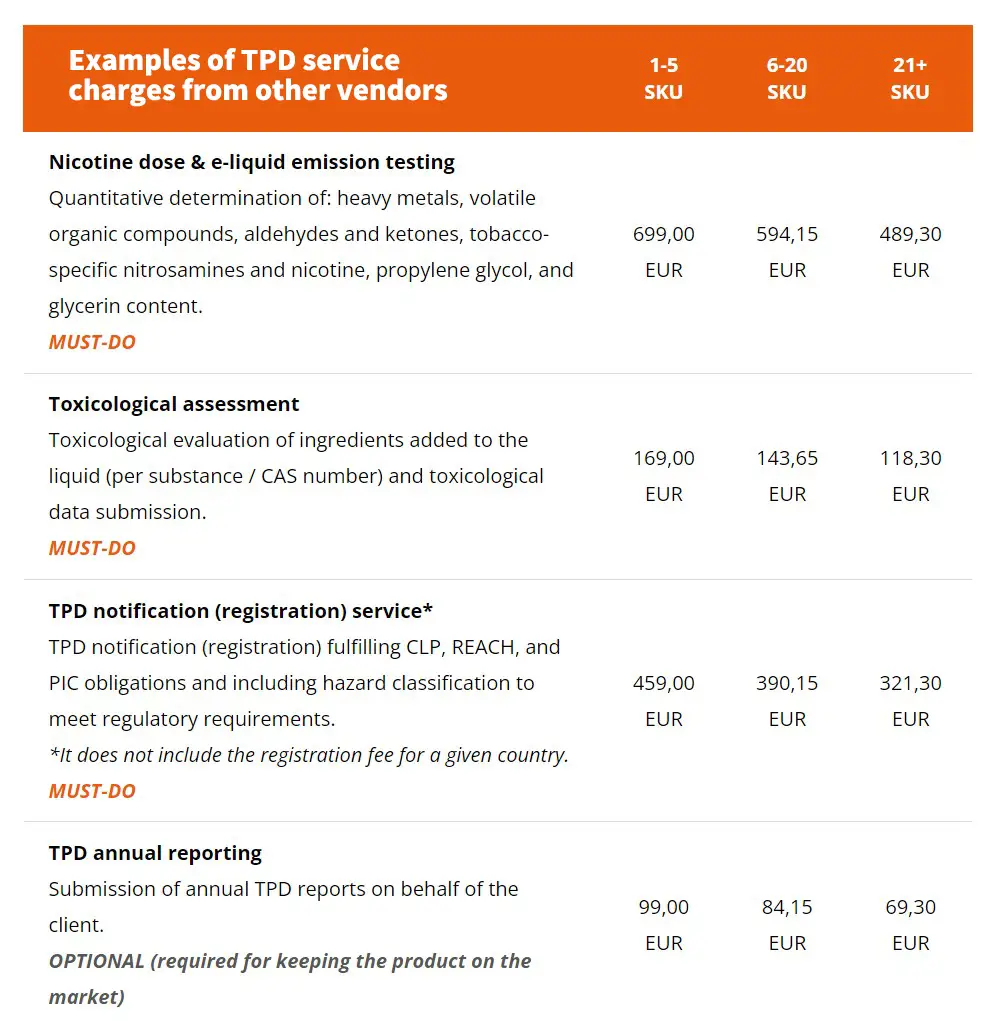
1.The notification shall, depending on whether the product is an electronic cigarette or a refill container, contain the following information:
(a) the name and contact details of the manufacturer, a responsible legal or natural person within the Union, and, if applicable, the importer into the Union;
(b) a list of all ingredients contained in, and emissions resulting from the use of, the product, by brand name and type, including quantities thereof;
(c) toxicological data regarding the product's ingredients and emissions, including when heated, referring in particular to their effects on the health of consumers when inhaled and taking into account, inter alia, any addictive effect;
(d) information on the nicotine doses and uptake when consumed under normal or reasonably foreseeable conditions;
(e) a description of the components of the product; including, where applicable, the opening and refill mechanism of the electronic cigarette or refill containers;
(f) a description of the production process, including whether it involves series production, and a declaration that the production process ensures conformity with the requirements of this Article;
(g) a declaration that the manufacturer and importer bear full responsibility for the quality and safety of the product, when placed on the market and used under normal or reasonably foreseeable conditions.
2.Eliquid and storage device related instructions:
(a) nicotine-containing liquid is only placed on the market in dedicated refill containers not exceeding a volume of 10 ml, in disposable electronic cigarettes or in single use cartridges and that the cartridges or tanks do not exceed a volume of 2 ml;
(b) the nicotine-containing liquid does not contain nicotine in excess of 20 mg/ml;

(c) the nicotine-containing liquid does not contain additives listed in Article 7(6);
(d) only ingredients of high purity are used in the manufacture of the nicotine-containing liquid. Substances other than the ingredients referred to in point (b) of the second subparagraph of paragraph 2 of this Article are only present in the nicotine-containing liquid in trace levels, if such traces are technically unavoidable during manufacture;
(e) except for nicotine, only ingredients are used in the nicotine-containing liquid that do not pose a risk to human health in heated or unheated form;
(f) electronic cigarettes deliver the nicotine doses at consistent levels under normal conditions of use;
(g) electronic cigarettes and refill containers are child- and tamper-proof, are protected against breakage and leakage and have a mechanism that ensures refilling without leakage.

3.Member States shall require manufacturers and importers of electronic cigarettes and refill containers to submit, annually, to the competent authorities:
(i) comprehensive data on sales volumes, by brand name and type of the product;
(ii) information on the preferences of various consumer groups, including young people, non-smokers and the main types of current users;
(iii) the mode of sale of the products;
(iv) executive summaries of any market surveys carried out in respect of the above, including an English translation thereof.
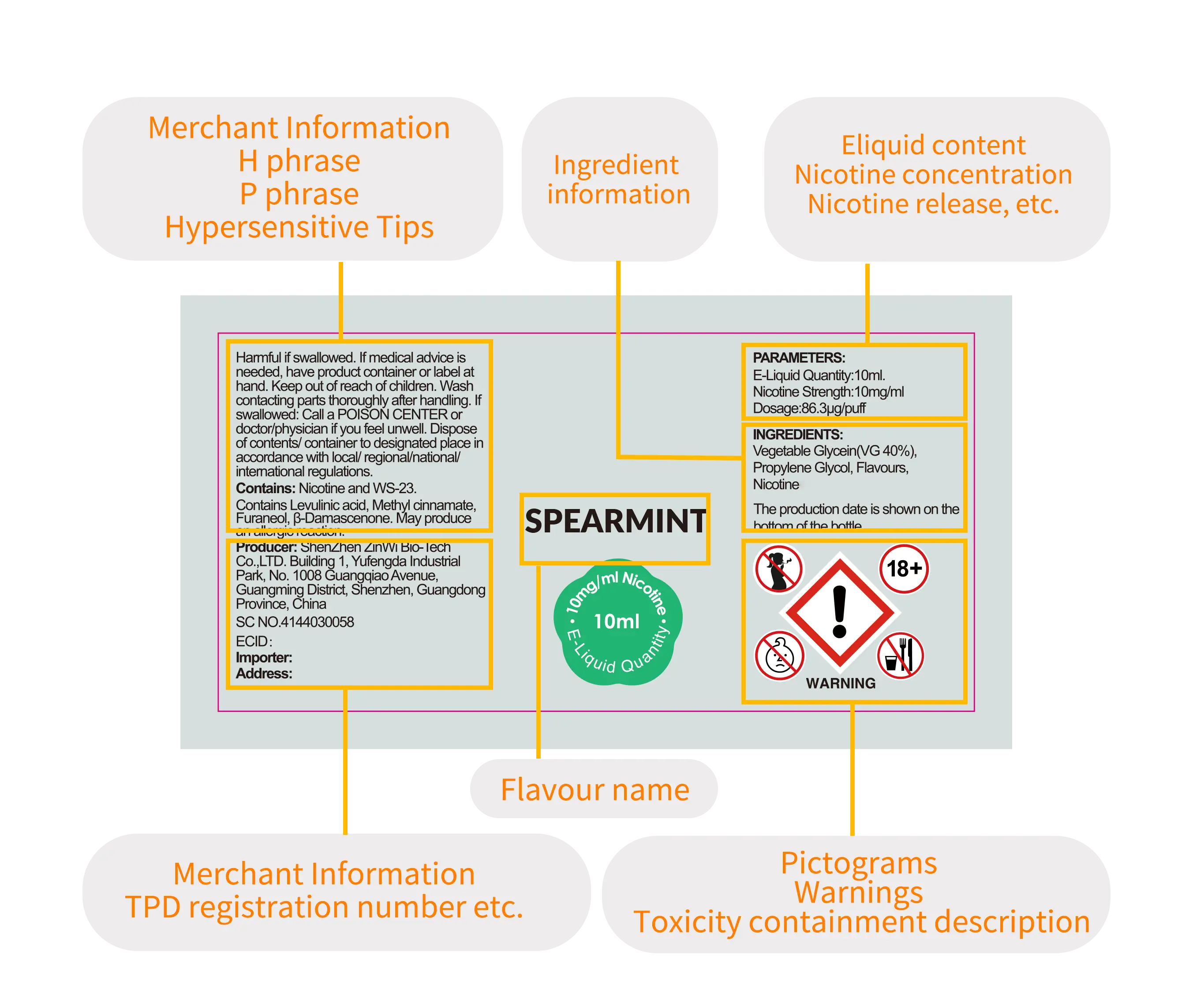
4.Labelling and Packaging Compliance Requirements:
TPD requires product packaging and labelling to provide clear information so that consumers can understand the contents and risks of the product. Labels need to indicate the product's ingredients, nicotine content, directions for use, and warning information to ensure that consumers can make informed choices.
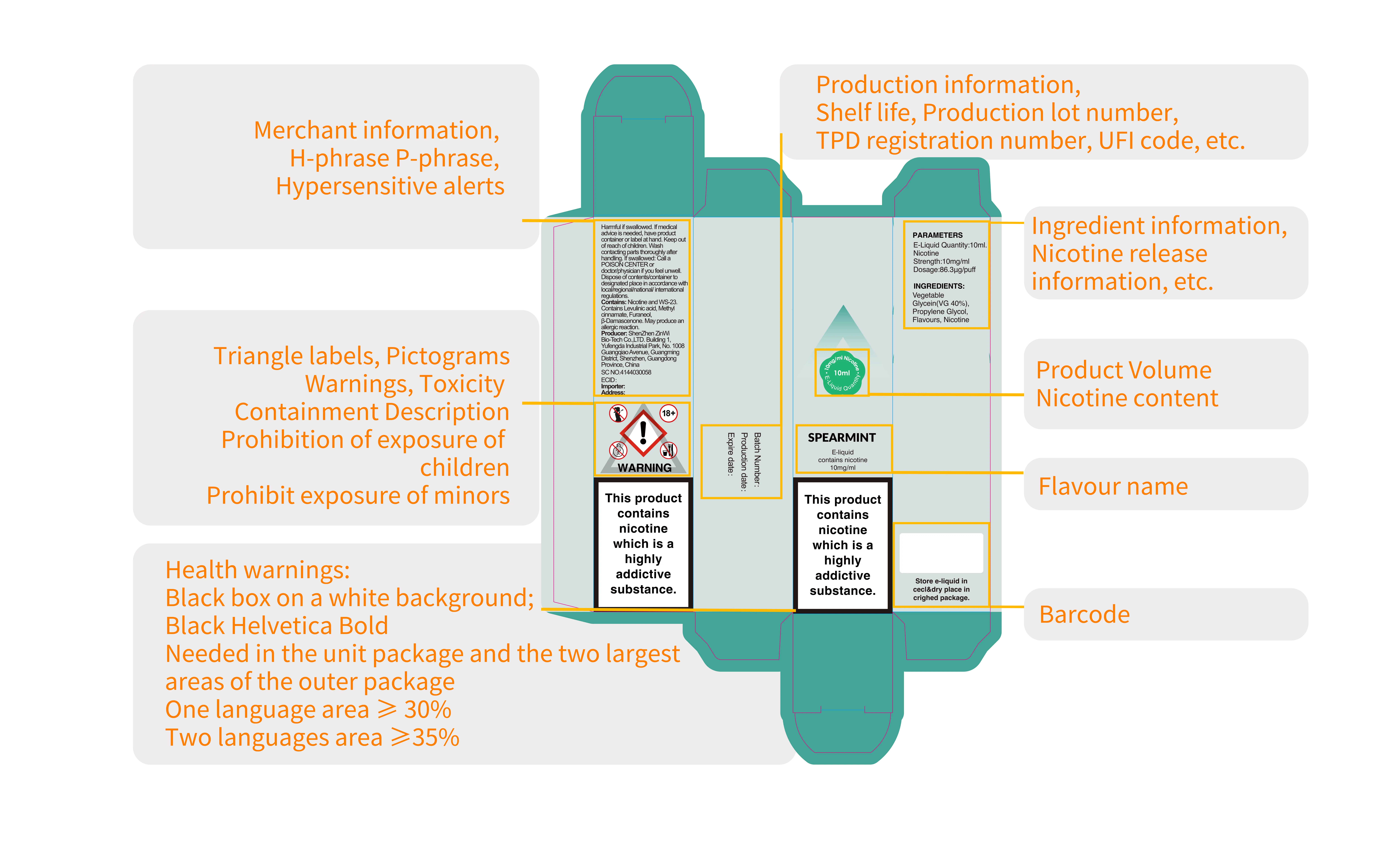
In addition to the TPD requirements, each of the 27 EU countries has its own additional regulations for e-cigarettes sold in their countries. Each country has detailed regulations on product packaging and warnings, personalised product certification, packaging container colour identification, time marking, website design, etc. For example, Denmark and Sweden will require that the product packaging colour can only be Pantone 448C or white unisex packaging.
5.Overview of the registration process
Registration for Electronic Cigarette Authentication (ECAS) in the EU TPD Directive;
Application for Submitter ID;
Form completion (approximately 10 working days to complete);
Notify the administrator by email after the registration is completed, stating that the ID registration is successful, with ECAS and Submitter ID;
After the success, log in the account opening information platform (EU-CEG) to fill in the information, upload attachments, and choose different countries to submit information respectively;
While TPD guidelines are largely consistent across Europe, individual member states may impose additional rules based on their unique health policies and regulations, such as specific prohibitions and promotional restrictions. Therefore, it is crucial to ensure that products comply not only with TPD requirements but also with the specific regulations of target market countries, while remaining vigilant about regulatory changes in each country.
In the face of the increasingly complex and ever-changing global regulatory landscape, Zinwi Bio has been dedicating substantial manpower and material resources to compliance and quality control since its inception. The company has established a robust team comprising seasoned compliance experts, legal advisors, and quality inspectors who boast extensive experience and are highly efficient and professional. These team members are well-versed in the various processes and requirements of EU TPD registration, backed by rich practical experience. Whether it's preparing product documentation, drafting technical files, or liaising with regulatory authorities, we are capable of providing clients with comprehensive professional support to ensure a smooth registration process.

Zinwi Bio utilises CNAS laboratories and conducts rigorous quality testing to ensure that each eliquid product complies with EU TPD standards and meets high quality control benchmarks. In addition, nicotine dosage and smoke emission testing, toxicological evaluations, and other related services are available.
To date, Zinwi Bio has successfully facilitated the registration of nearly 1,000 products for our customers, helping them reduce costs, enhance efficiency, and establish a dependable compliance assurance system with global partners.
"At Zinwi Bio, we treat compliance with the same rigor as product quality," stated the head of our compliance department. "We deeply understand the significance and challenges of TPD registration and recognize that compliance is indispensable for sustainability. That's why we not only provide high-quality electronic atomising fluids but also ensure regulatory compliance at every stage, from R&D to market. We take pride in assisting our partners in meeting global compliance challenges."

Zinwi Bio aspires to leverage its professional services to help clients forge more dependable and stable channels to the European market. With our competitive edge in professional expertise, we aim to assist clients in navigating the intricate global regulatory environment, thereby facilitating market penetration and propelling the entire industry towards enhanced compliance and superior quality. Choose us for professional compliance and broader market prospects!
We will contact you as soon as possible









